Shifting Focus from Cost to Value
Tuesday, 12 January, 2016
Health technology assessment is an evidence-based approach which is rapidly being adopted across the globe to help deal with the complex question of device procurement within hospitals. Dr Eugene Salole explains.
Oh, East is East and West is West, and never the twain shall meet…
In relation to new healthcare technology - with focus on cost-containment on the one hand and rising patient demand coupled with the desire for improved health outcomes on the other - Mr Kipling, the Mumbai-born English author, poet and Nobel Laureate’s cliché may seem to also hold true in the context of hospital budget-setting and purchasing decisions. Not necessarily so, however; in this sphere there continues to be steady progress towards engagement between the twain, as a result of a shift away from exclusive consideration of cost towards more focus on benefit delivered.
Hospital procurement officers in Australia face the same daunting issues as their counterparts in Canada, the UK and other developed nations with universal coverage (‘national health’) systems, in particular escalating costs, maintenance of a high quality of service provision and the continual availability of innovative, often expensive, technologies to help deliver care. Balancing access to potentially effective devices, which might enhance the delivery of quality care and improve patient outcomes, against setting (and not blowing) the hospital budget is a delicate and difficult task. At the heart of the dilemma is the issue of value: what benefits may the hospital or local purchasing group expect to receive, in terms of improved clinical outcomes, from investing in a particular class of technology or a specific product? An evidence-based approach which is rapidly being adopted across the globe to help deal with this complex question is ‘health technology assessment’ (HTA).
Health technology assessment (HTA)
In brief, HTA is a multidisciplinary research activity which aims to evaluate a new technology (or procedure or service) with the objective of providing decision-makers with robust recommendations upon which they may base adoption and funding decisions. It does this by explicitly gathering and evaluating the evidence around a product – the information available in support of the benefits claimed (clinical or otherwise), and also potential harms, and all the cost implications of adopting the technology (its likely utilisation rate, maintenance costs, etc.) – and then comparing these facets with the characteristics of the alternative products available [1]. This may appear to be no different from what usually happens when device procurement decisions are made - but the key distinctions with the HTA approach are that it is, first and foremost, data-driven; benefits (and potential harms) need to be substantiated by hard evidence, preferably from experimental studies like clinical trials, and all relevant costs identified.
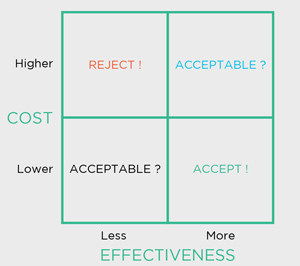
Secondly, and crucially, both the benefits and costs are then compared against those of a viable alternative, the ‘comparator’, which might be the product in current use or nothing at all (perhaps ‘usual care’ in a clinical setting). It is this explicit assessment of a new device technology against its comparator, in terms of both benefit and cost, that delivers the information from which its relative economic value, its cost-effectiveness, is gauged (Figure 1).
The evidence for benefits (and potential harms) of a technology is usually collected by researching the peer-reviewed literature, or may be supplied by the vendor (as is often the case when studies were not openly published, but available from the manufacturer as ‘whitepapers’), then appraised critically for quality and content, and finally the results summarised. In circumstances where data is available from several studies, the process of literature collection, review and summation is known as systematic review and meta-analysis, which usually concludes with an overall statistical point-estimate of the benefit as well as its range of uncertainty [2].
Usefulness of HTA
The economic evaluation component of HTA provides, at a minimum, the potential short-term (1-5 years) impact of purchase and adoption on the hospital budget; but it can also deliver an estimate of the economic value of the new product, its relative cost-effectiveness, in terms of dollars per tumour detected for example, or better, cost per successful health outcome, such as an additional year of survival gained. This figure not only frames the value of the new product but can (depending on the type of outcome measure used) also place it within a spectrum of cost-effectiveness for other technologies (even clinically unrelated ones), or procedures or services, on the basis of which rational purchasing decisions may be made across a broad range of options [3].
In principle, HTA can be applied to purchasing choices for any product, procedure or service; from a more user-friendly but more expensive handwash to a new piece of diagnostic imaging capital equipment. However, because undertaking HTA requires some expertise and is time consuming, its comprehensive form is best confined to situations involving significant expenditure or risk, and where uncertainty about the cost-effectiveness of the new versus the current is important, e.g., in Australia, applications to list on the Medicare Benefits Schedule (and thereby attract both government subsidy and private health insurance funding) new surgical procedures closely associated with innovative technologies involves going through a rigorous HTA process [4]. That said, so-called ‘mini-HTA’ or ‘rapid HTA reviews’, less expansive but more timely assessments, often undertaken to address specific questions, are much simpler projects and
have proved useful, particularly in the context of hospital procurement [5].
Looking ahead
HTA is not always easy to practise or always practicable [6], but its utility, philosophically and in practice, is its focus and reliance on evidence – thereby providing purchasers, policy-makers, healthcare providers, and also consumers, with a data-driven, transparent, contestable framework within which value-for-money discussions might be had and decisions made. For this reason, HTA has become installed on the global healthcare scene as a key tool to rationally control expenditure, and in many jurisdictions has been integrated as a formal part of the healthcare funding process (usually in relation to reimbursement at the patient level) – for instance, the Australian government has made it clear that HTA is key to achieving its overall objective of delivering a safe, effective and efficient health system that is also fiscally sustainable in the longer term [7].
However, some issues remain. Whereas ‘HTA thinking’ is well entrenched in relation to pharmaceuticals, on both the supplier and payer sides of the fence (e.g., it has been applied to the government subsidy of new medicines in Australia since at least 1993 [8]), in general the medical device and diagnostics sectors have lagged behind and continue to focus on selling specific features and benefits which purportedly differentiate one product from another, be it a stoma appliance or a sophisticated CT scanner, and not on substantiated health outcomes, the benefits which really matter [9, 10].
There are a range of reasons for this, including the extraordinary heterogeneity of medical technologies, an environment where rapid incremental innovations may result in product lifecycles as short as a few months, and the long time-lag before the true value (in terms of health outcomes) of surgically-implanted devices are realized [11]. However, things are changing - the worldwide concern about the sustainability of universal healthcare coverage in light of ageing populations, the epidemiological shift to chronic diseases and a shrinking tax base, as well as the current focus on quality of service, has turned attention towards device technologies and their relative value [12, 13].
In my view, the key recent development which will be a catalyst for change towards ‘HTA thinking’ in the devices and diagnostics sector is the lead shown by the CEO of a large US-based innovative device supplier, who has embraced the notion of value in healthcare and is enthusiastically steering the company down that path [14], making the likelihood that other medical technology suppliers will soon follow suit very high – and that would surely be a good thing, for all parties concerned.
Balancing access to potentially effective devices, which might enhance the delivery of quality care and improve patient outcomes, against setting (and not blowing) the hospital budget is a delicate and difficult task.
Figure: The ‘cost-effectiveness plane’: framework for making decisions based on clinical and economic value.

Further reading
National Health & Medical Research Council.
How to compare the costs and benefits: evaluation of the economic evidence. (Guide to clinical practice guidelines.) Canberra: NHMRC, 2001. Available at:
http://bit.ly/1lIFt2d accessed 24th September 2014.
 Eugene Salole
Eugene Salole
PhD MPH Principal, Value-Based Access Pty Ltd
Sydney - esalole@valuebasedaccess.com
References
- US National Library of Medicine. HTA 101: Introduction to Health Technology Assessment. Available at: https://www. nlm.nih.gov/nichsr/hta101/ta10103.html; accessed 29th October 2015.
- Bhandar M, Devereaux PJ, Montori V, Cinà C, Tandan V, Guyatt GH. Users’ guide to the surgical literature: how to use a systematic literature review and meta-analysis. J Can Chir 2004;47:60-67.
- Neumann PJ, Rosen AB, Weinstein MC. Medicare and cost- effectiveness analysis. N Engl J Med 2005;353 (14):1516-1522.
- Medical Services Advisory Committee. Final Protocol to guide the assessment of 18F-FDG PET in proven locally advanced, suspected locally and regionally recurrent, and suspected metastatic breast cancers. Application 1357. Canberra: 2014. Available at: http://www.msac.gov.au/internet/msac/publishing.nsf/Content/DE2732E8893DB5 A3CA257B810027B0B0/$File/1357-FDG%20PET-Final%20 Protocol%20%28D14-1548856%29%20%28D14-1567217%29. PDF; accessed 9th March 2015.
- Gagnon M-P. Opportunities to promote efficiency in hospital decision-making through the use of health technology assessment. Ottawa: Canadian Health Services Research Foundation; 2011. Available at: http://www.cfhi- fcass.ca/sf-docs/default-source/commissioned-research- reports/Gagnon-Dec2011-EN.pdf?sfvrsn=0; accessed 26th October 2015.
- Grobler M, Salole E The global push for health technology assessment: potential impacts on innovation. Drug Information Association Global Forum 2011;3 (5):29-31.
- Department of Health. HTA Policy Framework. Available at: http://www.health.gov.au/internet/hta/publishing.nsf/Content/policy-1; accessed 26th October 2015.
- Hailey D. Health care technology in Australia. Health Policy 1994;30:23-72.
- Van den Steen D, Simoens S, Vanleene V, et al. HTA Stomamateriaal in België. KCE reports 21 A. Brussel: Federaal Kenniscentrum voor de Gezondheidszorg; 2005. Available at: https://kce.fgov.be/sites/default/files/page_ documents/d20051027327.pdf; accessed 7th June 2015.
- Hollingworth W, Jarvik JG. Technology assessment in radiology: putting the evidence in evidence-based radiology. Radiology 2007;244:31-38.
- Fraser AG, Daubert J-C, Van de Werf F, et al. Clinical evaluation of cardiovascular devices: principles, problems, and proposals for European regulatory reform. Eur Heart J 2011;32: 1673-1686.
- Robinson JC. Value-based purchasing for medical devices. Health Aff (Millwood) 2008;27 (6):1523-1531.
- Barbash GI, Glied SA. New technology and health care costs – the case of robot-assisted surgery. N Engl J Med 2010;363 (8):701-704.
- Medical Device and Diagnostic Industry. Value-based healthcare as seen by Medtronic’s CEO. Available at: http://www.mddionline.com/article/value-based- healthcare-seen-medtronics-ceo-11-24-14; accessed 29th October 2015.
Remote laundries target preventable disease in NT communities
A new community laundry has launched in Borroloola, part of a program seeking to curb preventable...
Eye care partnership looks to support First Nations optometrists
A new scholarship initiative will support Aboriginal and/or Torres Strait Islander optometrists...
A Day in the Life of a mobile optometrist
Linda Nguyen is the owner and founder of mobile optometrist practice Care Optometry and was a...




![[New Zealand] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89856/wfmedia_thumb/..jpg)
![[Australia] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89855/wfmedia_thumb/..jpg)




