PVC dressings — comparing the old with the new
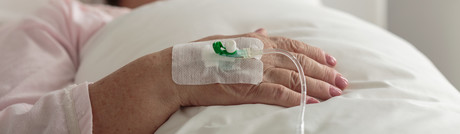
Peripheral venous catheters (PVC) are the most commonly inserted invasive device worldwide, with 2 billion purchased globally each year.1 PVC failure remains unacceptably high with up to 69% of devices failing due to dislodgement, phlebitis, occlusion, infiltration or infection.2 In part this is believed to be a result of poor securement of the catheter to the skin. There are numerous dressing and securement products available to clinicians but limited high-quality evidence to support the use of any one product.
This need was identified by the Alliance for Vascular Access Teaching and Research (AVATAR) Group, who have led a large multicentre randomised controlled trial comparing four dressing and securement options within two adult tertiary hospitals in Brisbane, Australia.
Studies products included:
- a simple polyurethane dressing;
- a bordered polyurethane dressing;
- a sutureless securement device with a polyurethane dressing; and a
- tissue adhesive with polyurethane dressing.3
In total, 1709 patients were enrolled to participate in the study between March 2013 and September 2014. The researchers hoped to compare how these dressing and securement options impacted on all-cause PVC failure.
The results demonstrated that, while overall patients allocated to the tissue adhesive had a lower rate of all-cause PVC failure (38%), compared with the bordered polyurethane (40%), sutureless securement (41%) and simple polyurethane (43%) groups, the impact was not statistically significant (p=0.21). Similarly, the time to failure was similar in all four groups (p=0.57).
Despite this, the study findings have demonstrated that PVC secured with tissue adhesive were less likely to fail specifically as a result of occlusion (5.6/100 PVC days; p=0.027) compared all other dressing and securement options (7.1–7.9/100 PVC days). So, should we be considering using tissue adhesive when placing all catheters? The answer to this may be determined by costs to the healthcare system.
To explore this, the researchers conducted an economic evaluation of each of the four treatment groups. Initial and replacement PVC dressings were significantly higher in the tissue adhesive group ($17.78) compared with the bordered polyurethane ($6.11), sutureless securement ($9.76) and simple polyurethane ($2.25) groups. This difference in mean costs was statistically significant, and may be a limiting factor for many institutions considering the use of this novel product.
An interesting and unexpected finding was the common use of dressing reinforcement by bedside clinicians (eg, non-sterile tapes; gauze and tubular bandaging) which most likely improved the performance of the four dressing and securement options. The use of reinforcements suggests an element of inherent mistrust of primary dressings applied to these PVC.
The researchers postulated that while tissue adhesive in combination with a simple polyurethane dressing (based on existing designs) may not benefit patients at a whole hospital population level, these products should continue to be considered for high risk and ‘difficult IV access’ patients. This recommendation is supported by other randomised controlled trials conducted to date which have demonstrated the benefits of tissue adhesives to secure PVC.4, 5
Further details are available on the Australian and New Zealand Clinical Trials Registry (ACTRN 12611000769987); ethics approval was obtained from the hospital ethics committee (HREC/11/QRCH/152) and Griffith University (NRS/46/11/HREC).
Patient experience with PVCs may improve with experienced vascular access specialistsPatients continue to experience multiple attempts (needle-sticks) for peripheral venous catheter (PVC) insertion, and device failure remains unacceptably high, according to the Alliance for Vascular Access Teaching and Research (AVATAR) Group. A recent study, conducted in a tertiary adult Brisbane hospital, was aimed at comparing PVC inserted by (i) a vascular access specialist (a clinician with advanced skills and knowledge) compared with (ii) generalist inserters (eg, bedside nurses and treating physicians).6 Patients were randomly allocated to one inserter type and followed up until either the device failed or was removed due to completion of therapy. In total, 138 medical and surgical patients were recruited to the study between July and November 2017. The researchers found 100% of devices were inserted after randomisation to a vascular access specialist. In contrast, 28% (19/69) of patients randomised to a generalist inserter never received a PVC within 24 hours of randomisation. For the majority of these patients, the PVC was simply cancelled, but it is unclear if this resulted from generalists lacking the required PVC insertion skill; or that a comprehensive assessment of the patient’s need for a PVC was not made by the treating medical team prior to requesting the device. Of particular concern, however, were three patients allocated to this generalist group who, after multiple ‘unpleasant’ insertion attempts by several staff, were still awaiting PVC placement 24 hours later. Overall the PVC failure rates in the VAS group were lower (48%) than the generalist group (54%); however, as expected with a pilot randomised controlled trials, the findings were not statistically significant. The cost and patient implications of a vascular access specialist model (compared with the current common model of generalist PVC insertion and care) are yet to be determined; however, these are likely to include such factors as:
The researchers concluded that a large multicentre randomised controlled trial comparing these two insertion models was necessary moving forward. Further details are available on the Australian and New Zealand Clinical Trials Registry (ACTRN12616001675415); ethics approval was obtained from the hospital ethics committee (HREC/16/QRBW/386) and Griffith University (2016/782). |
References
- Rickard, C.M. and G. Ray-Barruel, Peripheral intravenous catheter assessment: beyond phlebitis. The Lancet Haematology, 2017. 4(9): p. e402-e403.
- Bolton, D., Improving peripheral cannulation practice at an NHS Trust. British Journal of Nursing, 2010. 19(21): p. 1346-1350.
- Rickard, C.M., et al., Dressings and securements for the prevention of peripheral intravenous catheter failure in adults (SAVE): a pragmatic, randomised controlled, superiority trial. The Lancet, 2018. 392(10145): p. 419-430.
- Bugden, S., et al., Skin glue reduces the failure rate of emergency department–inserted peripheral intravenous catheters: A randomized controlled trial. Annals of Emergency Medicine, 2016. 68(2): p. 196-201.
- Marsh, N., et al., Securement methods for peripheral venous catheters to prevent failure: a randomised controlled pilot trial. The Journal of Vascular Access, 201 16(3): p. 237-244.
- Marsh, N., et al., Expert versus generalist inserters for peripheral intravenous catheter insertion: a pilot randomised controlled trial. Trials, 2018. 19(1): p. 564.
Patients co-design invasive heart surgery monitoring clinical trial
Patients and their families have co-designed a clinical trial to determine if invasive devices...
Have Australian researchers discovered the secret to treating sepsis?
Promising results from a Phase 2 clinical trial have Australian researchers looking to progress...
Could this tailored heart pump transform care for half of heart failure patients?
Half of the 64 million people living with heart failure have no access to heart pump treatments....

![[New Zealand] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89856/wfmedia_thumb/..jpg)
![[Australia] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89855/wfmedia_thumb/..jpg)




