Tracking products to patients to improve patient safety
We can trace an apple back to its grower, but can’t track medical products throughout the supply chain to patients. Traceability needs to be a focus of our healthcare system.
As patients in our health system, we expect the same level of product traceability that is becoming the norm for the consumer products and food that we buy. We can now scan an individual piece of fruit and the processes used and available data enables us to track its origin back to the grower. But are we able to track products through the supply chain all the way to patients across our healthcare system? The answer today is no. So why haven’t we introduced these concepts in the health system to improve our safety, information and experiences as Australian health consumers?
Think like a healthcare consumer
If we step outside our organisational lenses for a minute and think like the health consumers that we are, we probably assume that our health system already has the basic technology to support traceability and track all patient activities in real time.
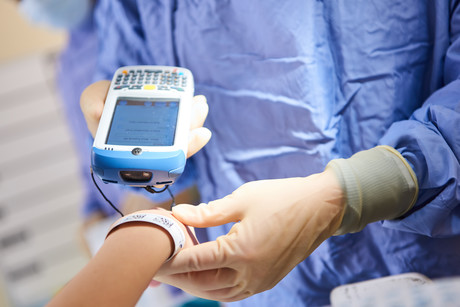
Technologies like blockchain, artificial intelligence (AI) and the Internet of Things (IoT) are the new tech buzzwords that have captured our attention. What you may not know is that the data interoperability and data capture that is needed to make these new technologies a success starts with technology basics such as unique identification and barcode scanning at point of care.

Today, the use of unique identification to ensure ‘positive patient identification’ across point of care activities and the technology to capture this data is limited in Australian hospitals. Likewise, our ability to automatically capture unique identification to ensure we have visibility of what products are available in our hospitals, where they have come from, whether they are safe to use on patients and which products are administered in real time to patients is also limited. We are not alone, as countries such as the United Kingdom have also been developing a national program to address these issues.
|
The UK Secretary of State for Health and Social Care, The Rt Hon Matt Hancock, gave a recent speech to the UK’s Royal Society of Medicine where he highlighted the need for the right technology to help clinical staff to carry out their jobs safely and efficiently. Comparing the NHS to Tesco he said: “Right now, Tesco has more sophisticated and more efficient systems than the NHS.” He continued, saying: “They can run their operations with just-in-time deliveries and market their goods to shoppers with personalised discount vouchers. “In the NHS, we don’t have anything like that. We don’t use common identifiers to identify patients, we don’t know which hospitals a patient has been to, we don’t know which medicines have been put into them. We don’t even know what we already know!” |
Opportunities provided by regulatory changes
Globally, regulations introduced to safeguard patients and consumers, such as the European Falsified Medicines Directive, or the many related to Unique Device Identification (UDI), should encourage us to look at the opportunities these milestones provide for our health system to improve traceability. Additionally, there are potential benefits to initiatives such as Australia’s My Health Record.
As a result of overseas developments, there are already opportunities for Australian health care to improve traceability, particularly with the emergence of unique identification on products.
As the Australian journey continues to a future ‘track & trace’ of pharmaceuticals or a possible ‘UDI’ system via industry consultations — largely led by the Therapeutic Goods Administration (TGA) — we should start thinking about the technology that will be needed across the health system to reap the benefits for all Australians. This is critical to ensuring that we can take advantage of the changes that any new regulations deliver.
Looking beyond the rounds of consultations, new regulatory measures and the deadlines for compliance which are impacting healthcare manufacturing communities, it is important for all the stakeholders to fully understand and engage in the change process. In doing so we can realise the many downstream benefits resulting from new regulatory measures. This will in turn support real change in how we deliver and manage our health systems and capture data for health records.
Indeed, thanks to regulatory change, other countries which are treading the path ahead of us are already seeing improved patient safety and data accuracy in acute care settings. Further, we are seeing the introduction of consumer health self-management capabilities, plus operational efficiencies in supply chain-related processes.
As a result, planning for and investing in technology and process changes — in parallel with the regulations being developed — is critical to delivering end-to-end visibility and traceability through to the patient.

Supporting traceability
Implementing a framework of unique identification to support traceability to the patient is a logical starting point. Within our hospitals we can then start implementing core data capture technologies and then add the more complex, intelligent technologies such as AI to help create even more efficiencies. We always have other priorities for our health budgets as we have needed to invest in major infrastructure; however, additional investment in enabling technologies will help to deliver more from all of our facilities.
While not without its challenges, a traceability framework enabled through unique identification and investment in technology will have many benefits for clinicians, health providers and ultimately, Australian health consumers. We should all be focused on this goal.

Some patients wait 6 years to see a public hospital specialist. Here's how to fix this
Australian research spanning more than a decade suggests there are ways to reduce waiting lists...
Reusing medical equipment is good for the planet. But is it safe?
A new study tested replacing just one kind of item — single-use absorbent pads, known...
Stewardship and sustainability: environmentally minded medical products
Australia's health sector produces twice as much carbon emissions as aviation. But with...




![[New Zealand] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89856/wfmedia_thumb/..jpg)
![[Australia] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89855/wfmedia_thumb/..jpg)




