Securing trust in the global vaccine supply chain

Billions of vaccines are needed urgently across the world. How can demand be met quickly, efficiently and securely?
It is fundamental to the success of current vaccination efforts that citizens trust the process and feel protected. According to a recent publication from Deloitte, concerted effort is necessary in four key areas to achieve efficiency, security, speed and public trust in vaccine delivery.
1. Advancing industry collaboration
Vaccination on a global scale requires common effort between industry, scientists and international organisations. Pharmaceutical companies are not in competition with one another on the development of a vaccine; instead, they are working in collaboration with the world’s top scientists to save lives. These partnerships, both in vaccine development and in clinical trials between industry and universities, create trust.
2. Embracing global standards for supply chain security
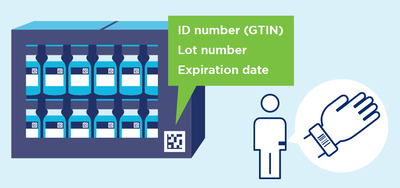
Vaccines, therapeutics, and associated medical devices and consumables present an urgent need for traceability built upon globally identified products. Using existing global supply chain standards for harmonised implementation of regulatory requirements will further patient safety goals, adding an element of trust at all levels of the supply chain. GS1 data standards provide additional visibility and traceability in these critical supply chains through unique identification, data capture and data sharing for shipments, locations, parties, individuals and critical events in an interoperable way.
Every product, at every level of packaging, is uniquely identified and this information is captured in a standardised barcode that can be read by all supply chain partners and is essential for health care providers to administer vaccines with confidence. WHO has recommended, a DataMatrix should be applied on secondary packaging (carton boxes), and — if possible — also on primary packaging (vial or prefilled syringe).
3. Anticipating challenges for safe and efficacious delivery of vaccines
Globally unique identification and barcoding of vaccines is critical for clinical trials, distribution, and pharmacovigilance. It is important to identify and label the vaccines, capturing precisely which patient received which vaccine, and when.
Challenges to anticipate include capturing adverse events, falsified vaccines, cold chain requirements for administration sites, trust of vulnerable populations most impacted by the virus, and mixing and matching of vaccines depending on availability.
4. Using clear and transparent communication to build vaccine confidence
Vaccine uptake will need to be facilitated by clear, evidence-based, and tested communications. It is critical that governments and the private sector come together to build confidence and ensure that patients have trust in the newly developed vaccines — especially since vaccination may be voluntary in many parts of the world.
“As the world gears up for the largest deployment of vaccines in history, it is more important than ever that supply chains are up to the task of maintaining trust and ensuring effective, timely delivery. We need to be able to trace every vaccine dose — from shipping to delivery and finally administration — and we need better adoption of common standards.” — Dr. Seth Berkley, Chief Executive Officer, Gavi.
Click here to access the Deloitte white paper.
Everyone deserves to be safe. Meeting this challenge depends on all of us.
Global standards help to ensure supply chain security, increase patient safety, and provide trust in the vaccines, medicines, and medical products distributed all around the world.
For more information about GS1 standards in healthcare contact healthcareteam@gs1au.org, visit www.GS1au.org/healthcare or call 1300 227 263.
Enhancing Patient Care Through Smart Design at Paula Fox Melanoma & Cancer Centre
GENTEC worked closely with the contractors to supply solutions for clinical areas where hygiene...
Revolutionising healthcare on-premise laundry
Alongside peristaltic, solenoid and pneumatic pump-driven equipment, SEKO has revolutionised...
Why Electrical Services Under HSV Contracts Are Vital for Healthcare
Behind every theatre, emergency room, and ward lies a network of electrical systems that...





![[New Zealand] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89856/wfmedia_thumb/..jpg)
![[Australia] Transform from Security Awareness to a Security Culture: A Vital Shift for SMB Healthcare — Webinar](https://d1v1e13ebw3o15.cloudfront.net/data/89855/wfmedia_thumb/..jpg)




