Method tricks stem cells into becoming bone
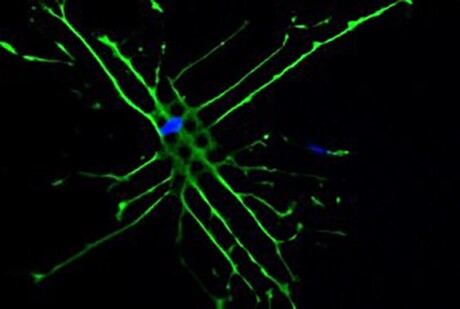
An international research team led by Monash University has found a way to alter the shape and nucleus of individual stem cells, which could speed up recovery following bone replacements. The findings are published in Advanced Science.
The research collaboration, involving Monash University, the Melbourne Centre for Nanofabrication, CSIRO, the Max Planck Institute for Medical Research and the Swiss Federal Institute of Technology in Lausanne, developed micropillar arrays using UV nanoimprint lithography that essentially ‘trick’ the cells into becoming bone.
Nanoimprint lithography allows low-cost, high-throughput and high-resolution microscale patterns to be created. When implanted into the body as part of a bone replacement procedure, such as a hip or knee, researchers found these pillars — which are 10 times smaller than the width of a human hair — changed the shape, nucleus and genetic material inside stem cells.
The team defined the topography of the pillar sizes and the effects it had on stem cells, discovering that four times as much bone could be produced compared with current methods.
“What this means is, with further testing, we can speed up the process of locking bone replacements with surrounding tissue, in addition to reducing the risks of infection,” said Associate Professor Jessica Frith from Monash University’s Department of Materials Science and Engineering.
“We’ve also been able to determine what form these pillar structures take and what size they need to be in order to facilitate the changes to each stem cell, and select one that works best for the application.”
Researchers are now advancing this study into animal model testing to see how they perform on medical implants.
Engineers, scientists and medical professionals have known for some time that cells can take complex mechanical cues from the microenvironment, which in turn influences their development. However, Dr Victor Cadarso from Monash University’s Department of Mechanical and Aerospace Engineering says their results point to a previously undefined mechanism where ‘mechanotransductory signalling’ can be harnessed using microtopographies for future clinical settings.
“Harnessing surface microtopography instead of biological factor supplementation to direct cell fate has far-reaching ramifications for smart cell cultureware in stem cell technologies and cell therapy, as well as for the design of smart implant materials with enhanced osteo-inductive capacity,” Dr Cadarso said.
Professor Nicolas Voelcker from the Monash Institute of Pharmaceutical Sciences, who is also Director of the Melbourne Centre for Nanofabrication, said the study results confirm that micropillars not only impact the overall nuclear shape, but also change the contents of the nucleus.
“The ability to control the degree of deformation of the nucleus by specifying the architecture of the underlying substrate may open new opportunities to regulate gene expression and subsequent cell fate,” Professor Voelcker said.
Virus samples missing from Qld lab, investigation launched
Queensland Health will conduct an Investigation into a major breach at the state's Virology...
Small weight gains can increase back pain, disability risk
A study linking body composition and back pain in men has found that as little as three kilograms...
Certain hormone treatments linked to increased heart disease risk
Specific hormone replacement therapy (HRT) treatments involving both oestrogen and progestogen...









